8D reports
Targeted management of quality deviations for accredited laboratories.
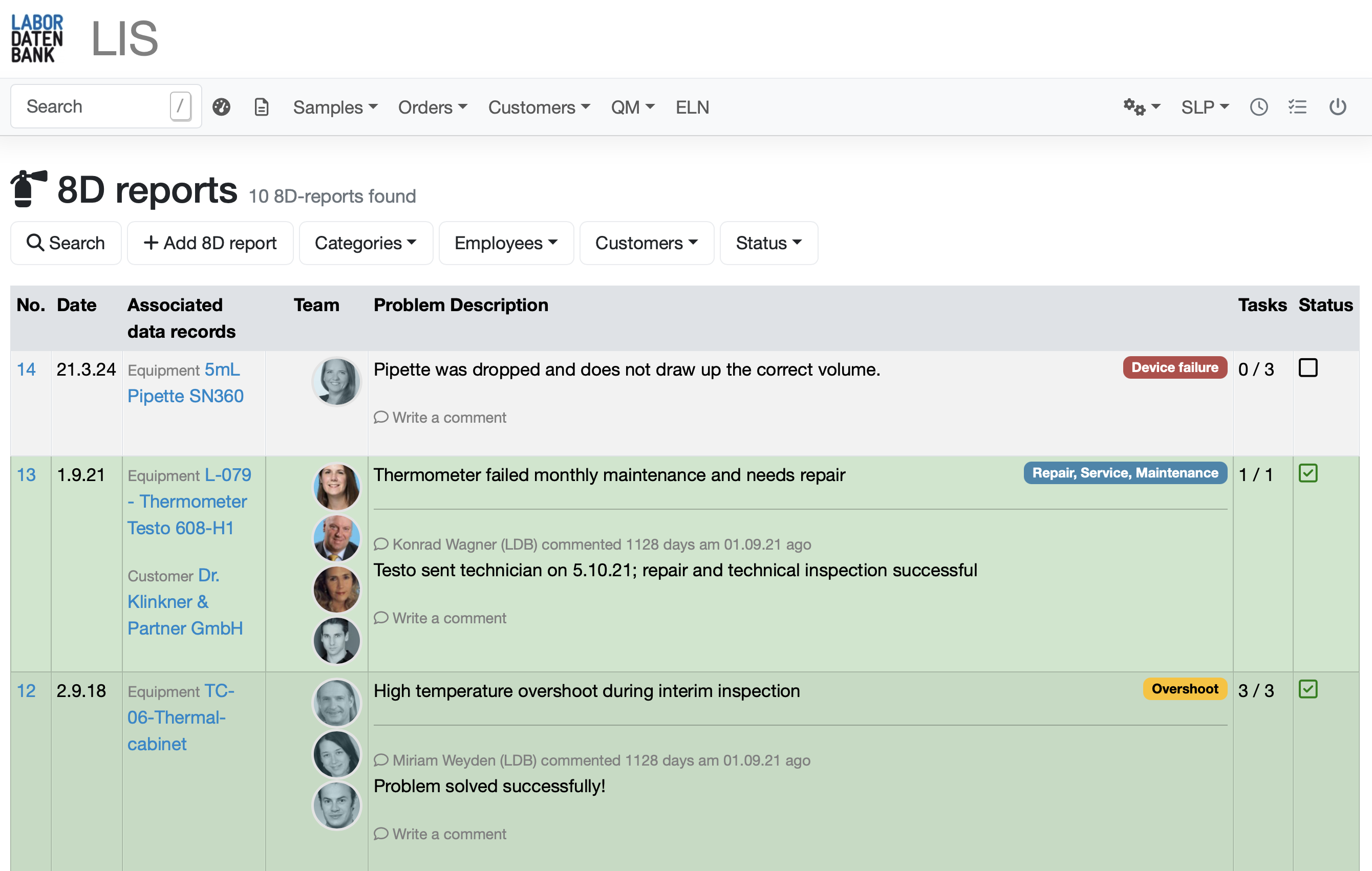
- Systematic identification
Detect and document quality deviations accurately and efficiently. Immediately with every data set. - Comprehensive documentation
Every deviation is recorded in detail, including root cause analysis and affected processes. This provides you with complete documentation and a history of all incidents and measures for audits. - Initiate corrective measures
Implement and track corrective measures directly in the system. - Check effectiveness
Evaluate the effectiveness of the measures taken to ensure that deviations are not repeated. - Transparent communication
Automatically inform affected parties from your team about the measures taken. Don't forget to acknowledge the team's performance!
LIMS functions
Laboratory Information Management System (LIMS)
- DashboardUpdate
- Sample management
- Plan orders & samples
- Import interfaces
- Customer management
- Customer zone
- LIMS validation
- Billingincl. e-bill
- Statistics & evaluations
- Test reports
- Access rights
- Audit Trail
QM modules
Simplify and automate documentation

