For ISO 17025 & GxP Pharmaceutical Labs
QM & System Validation – Fully Integrated
Flexible APIs for devices and external systems
21 CFR Part 11 Compliant Records & Digital Signatures
EU-hosted & ISO 27001 certified data security




220+ customers
in 6 different industries
5,000+ users
in 12 countries across Europe & the US
15+ years of experience
from the best labs in Europe
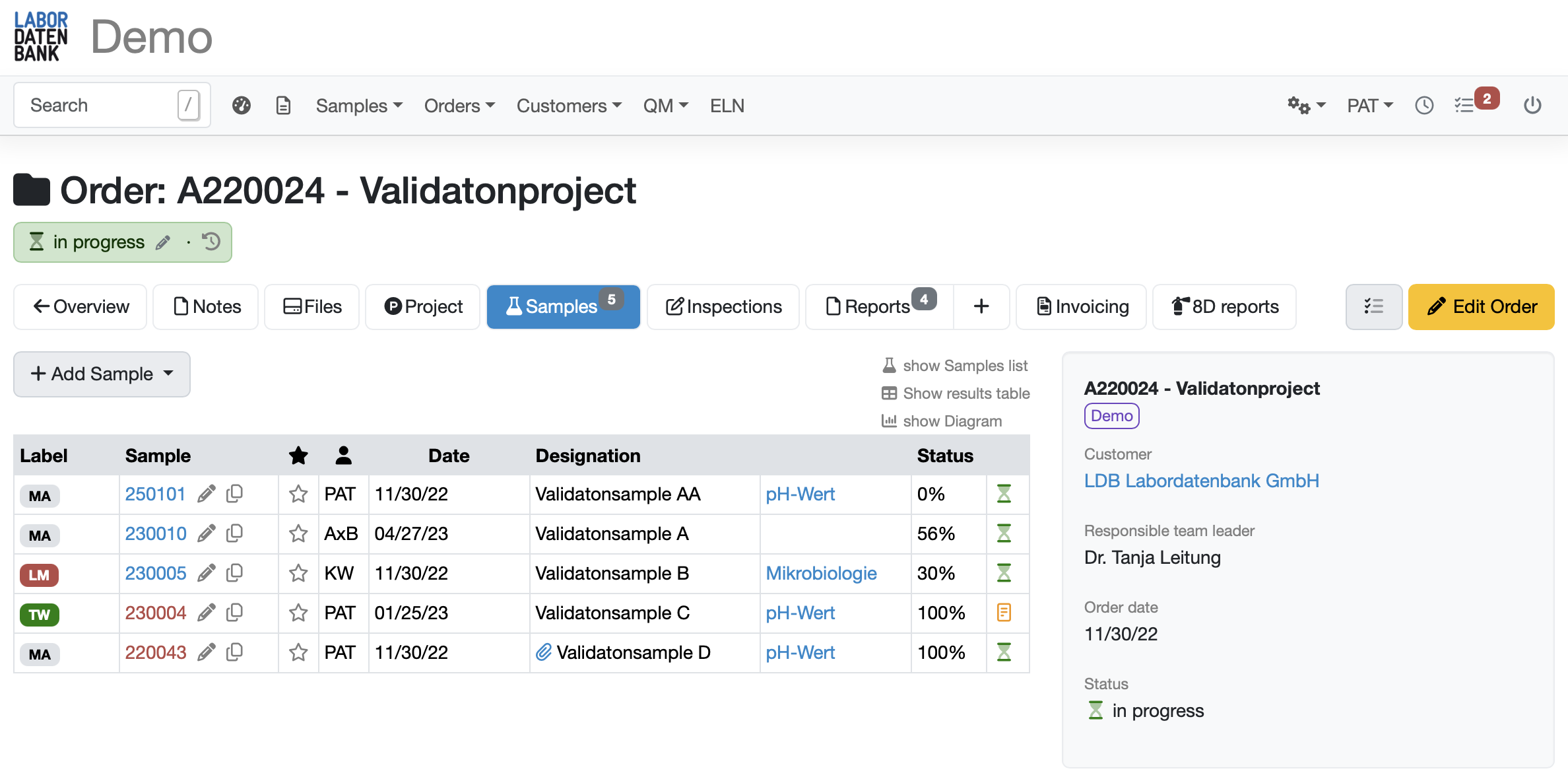
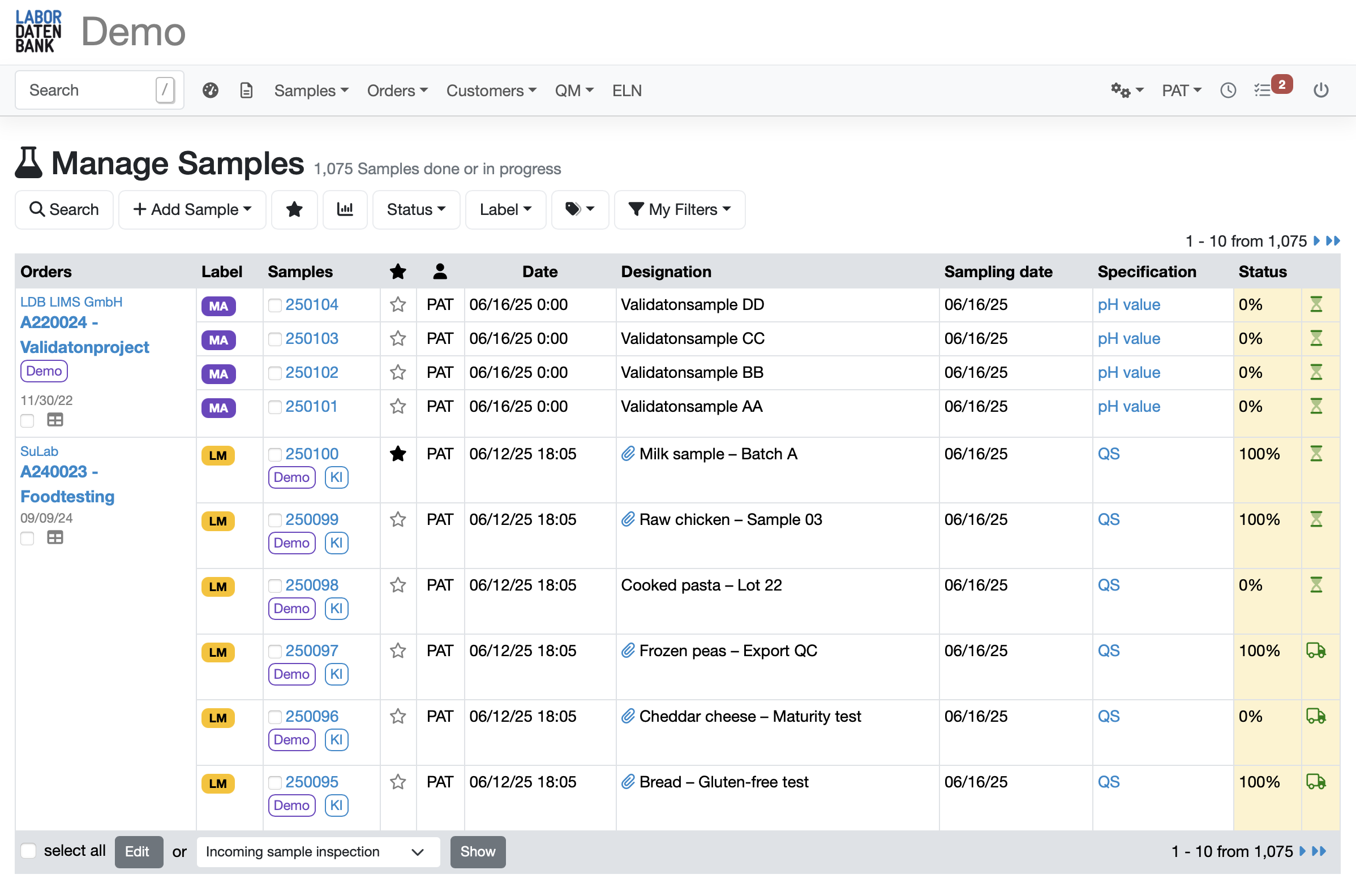
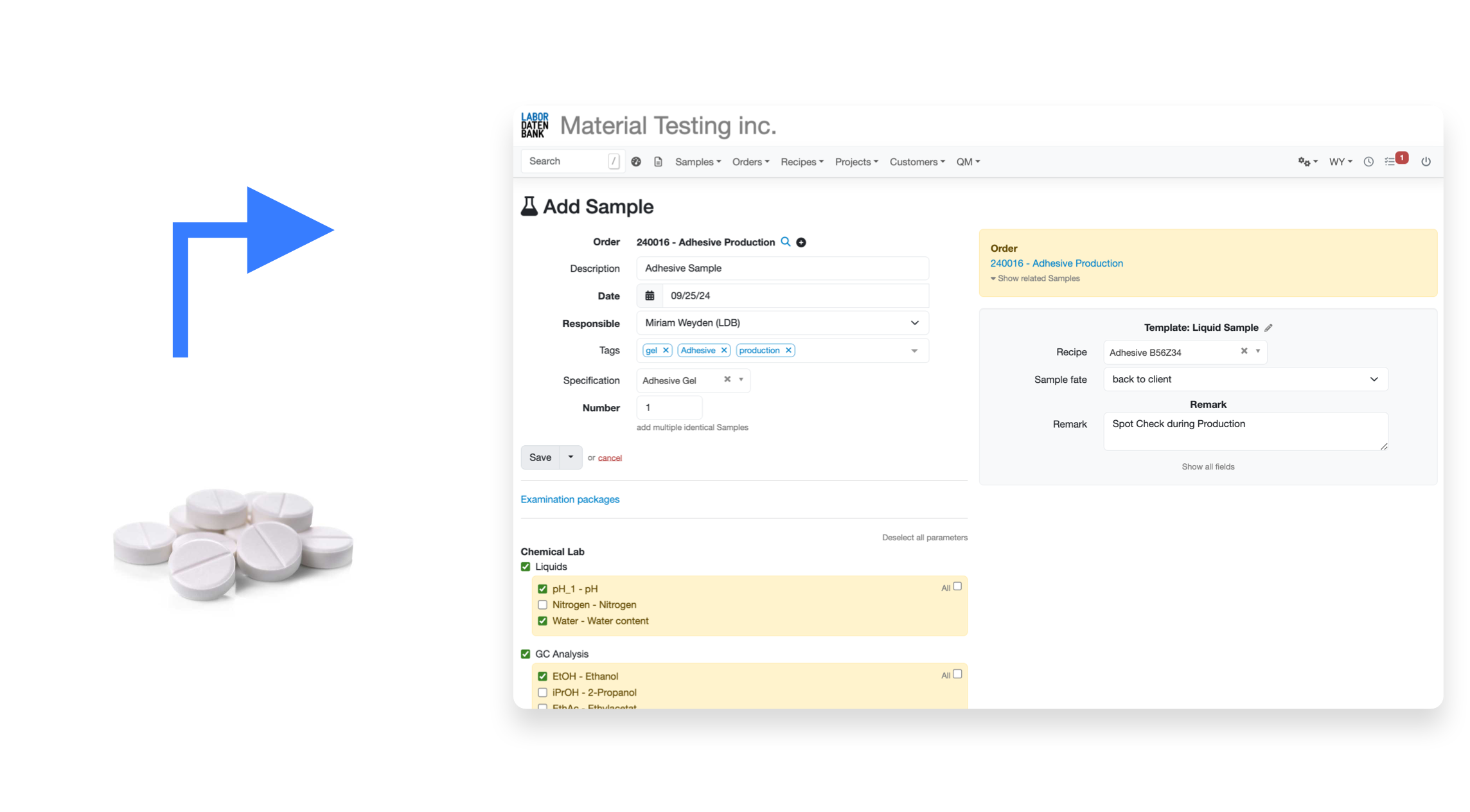
Simplify sample registration with flexible APIs & sample templates for different analyses
-
Comprehensive templates for different sample types
with pre-populated parameters and sample information
-
Grouping of connected parameters and analyses
for quick and easy selection of complex analyses across multiple parameters and measurements
-
Flexible customization of templates on the go
conviniently add additional analyses and information to each sample if needed
-
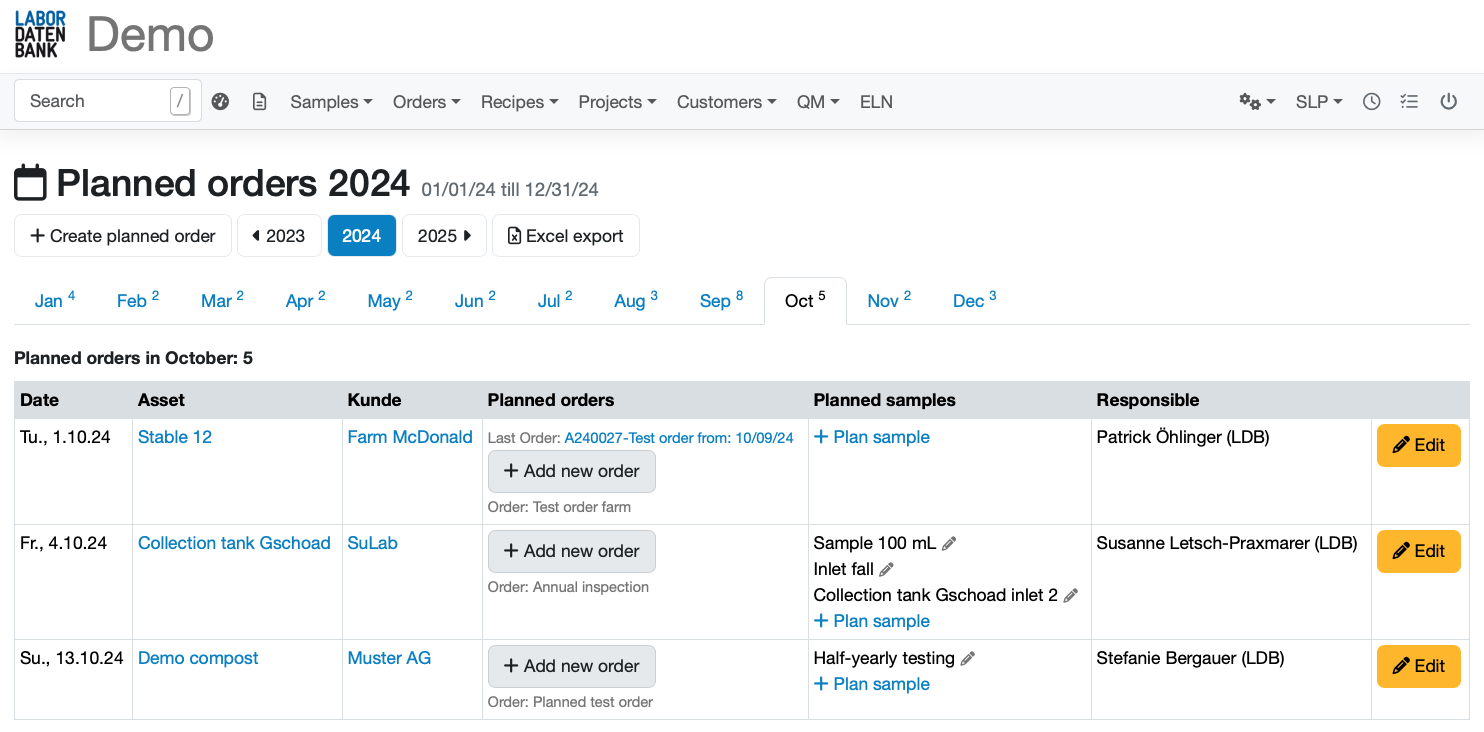
Automatically generate work lists for each sample
that reflect the specific tasks and requirements of each individual analysis

You want to learn more about sample intake?
Book a free call with our experts:
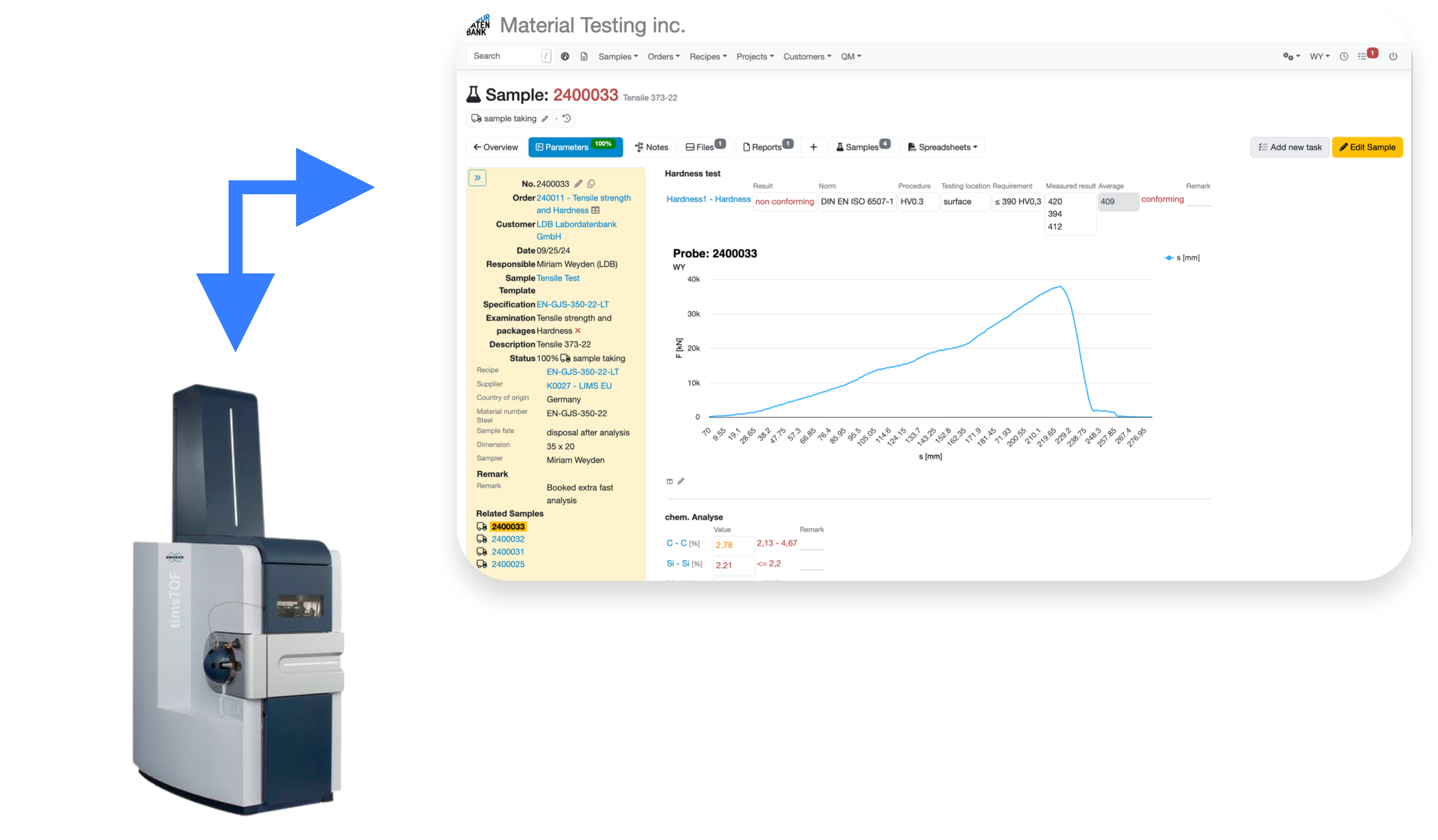
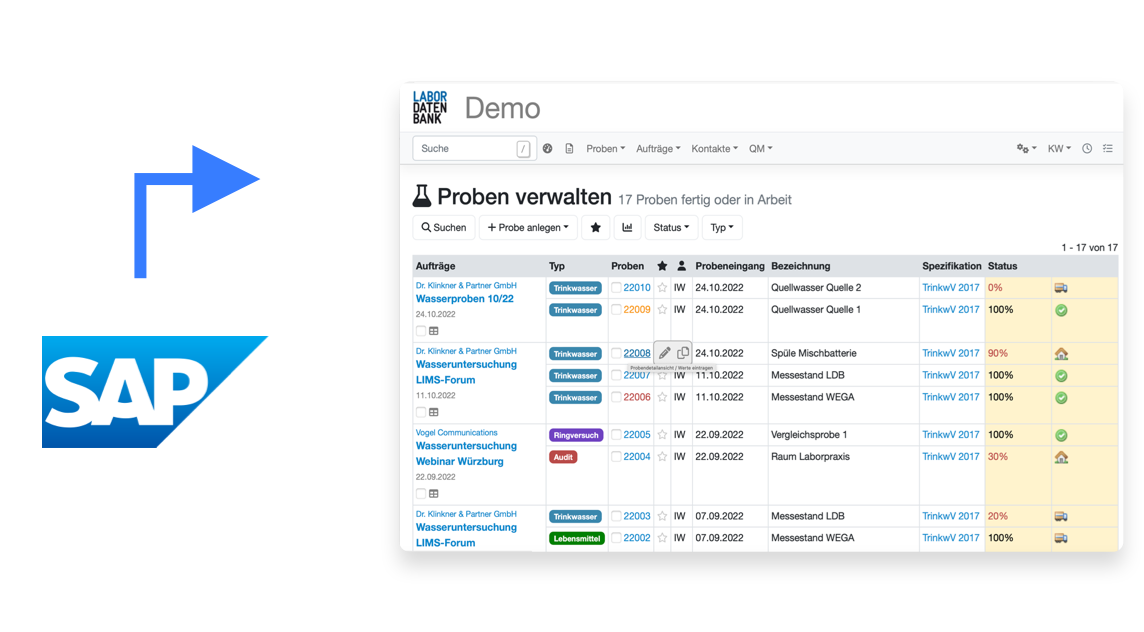
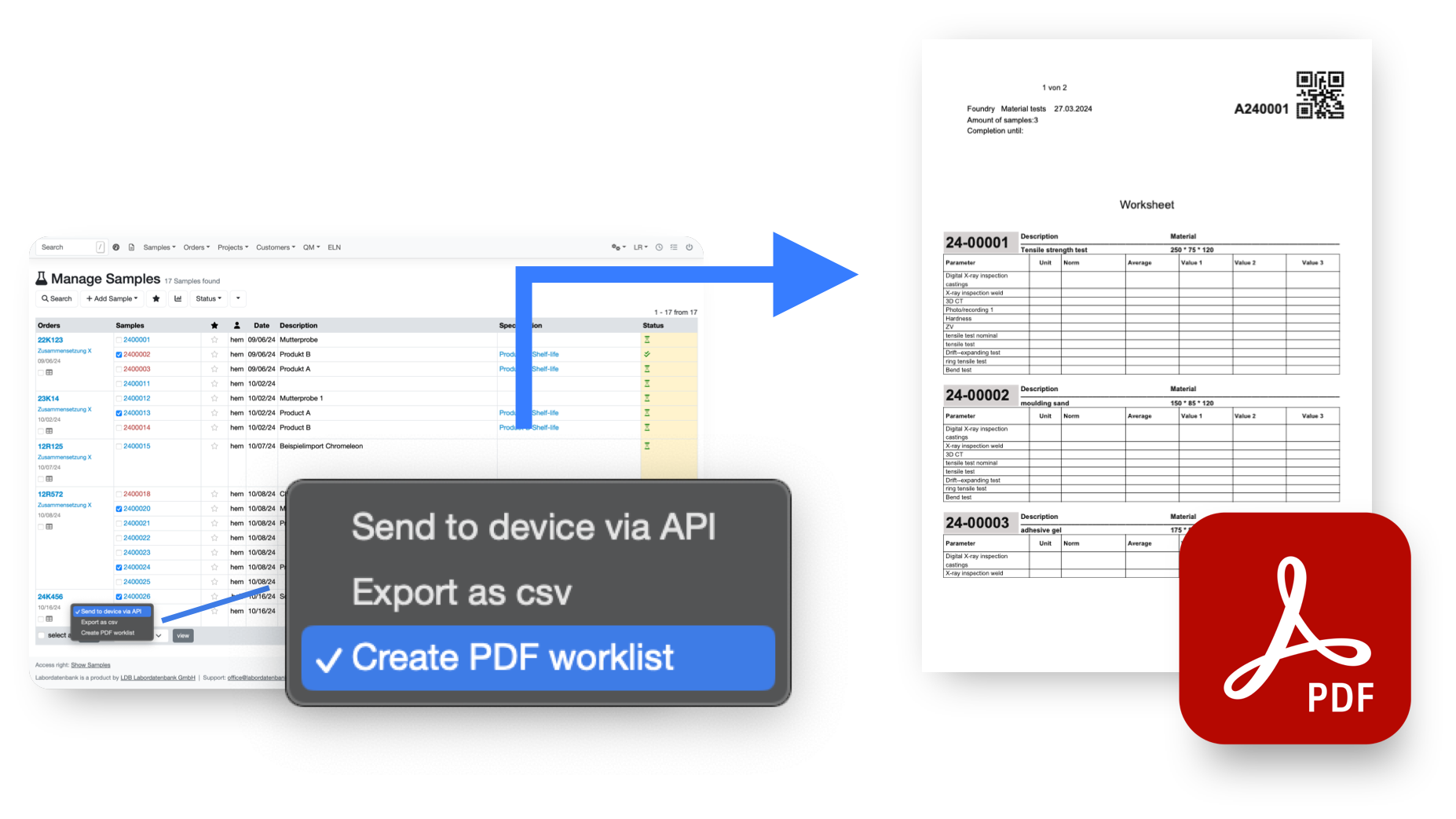
Transfer data to any device
-
Send your sample data directly to the device
via flexible and fully customizable APIs that can connect to all of your network enabled devices
-
Export device specific files
to import on your analyzing devices without any manual data input
-
Create worksheets specific to your workflows
to support manual data collection for processes that can not be digitized


You want to learn more about device connectivity options?
Book a free call with our experts:
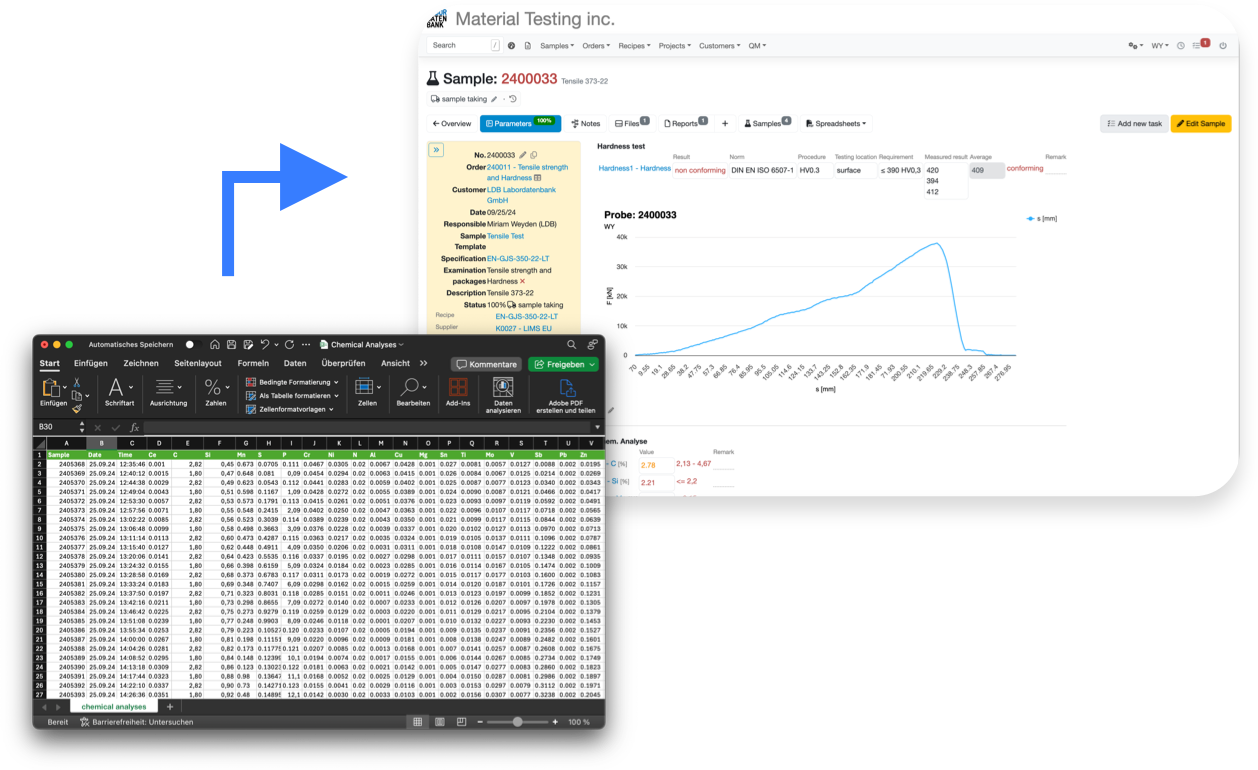
Submit your results via flexible and fully customizable
file imports & APIs
-
Alleviates need for 4 eyes principle
Once the API has been validated, all results are automatically considered to be validated in accordance with the GxP standards as well.
-
Easy to set up
Data exchange via FTP, HTTP or even Mail available at the click of a button
-
PUSH and PULL endpoints available
Data can be pushed by the device or fetched from the device, depending on your needs
-
Secure transmission
Requests can be secured via credentials, token authentication or P12 certificats
-
No programming required
Sample numbers are assigned according to individual requirements in accordance with the accreditation specifications
-
Supports all relevant file formats
import csv, json, txt, hl7, xml, fhir, gdt, chromelion, animl, shapth and many more. As long as it contains text data, LIMS.eu has you covered
-
Fully customizable data structure
you can freely structure your data as you see fit, LIMS.eu can handle it
-
Transformation of imported data
if your instrument provides data that is not structured in a way that is suitable, you can freely transform it using the whole power of javascript
-
Input from Anywhere
using any pc, tablet or phone right where the analysis happens
-
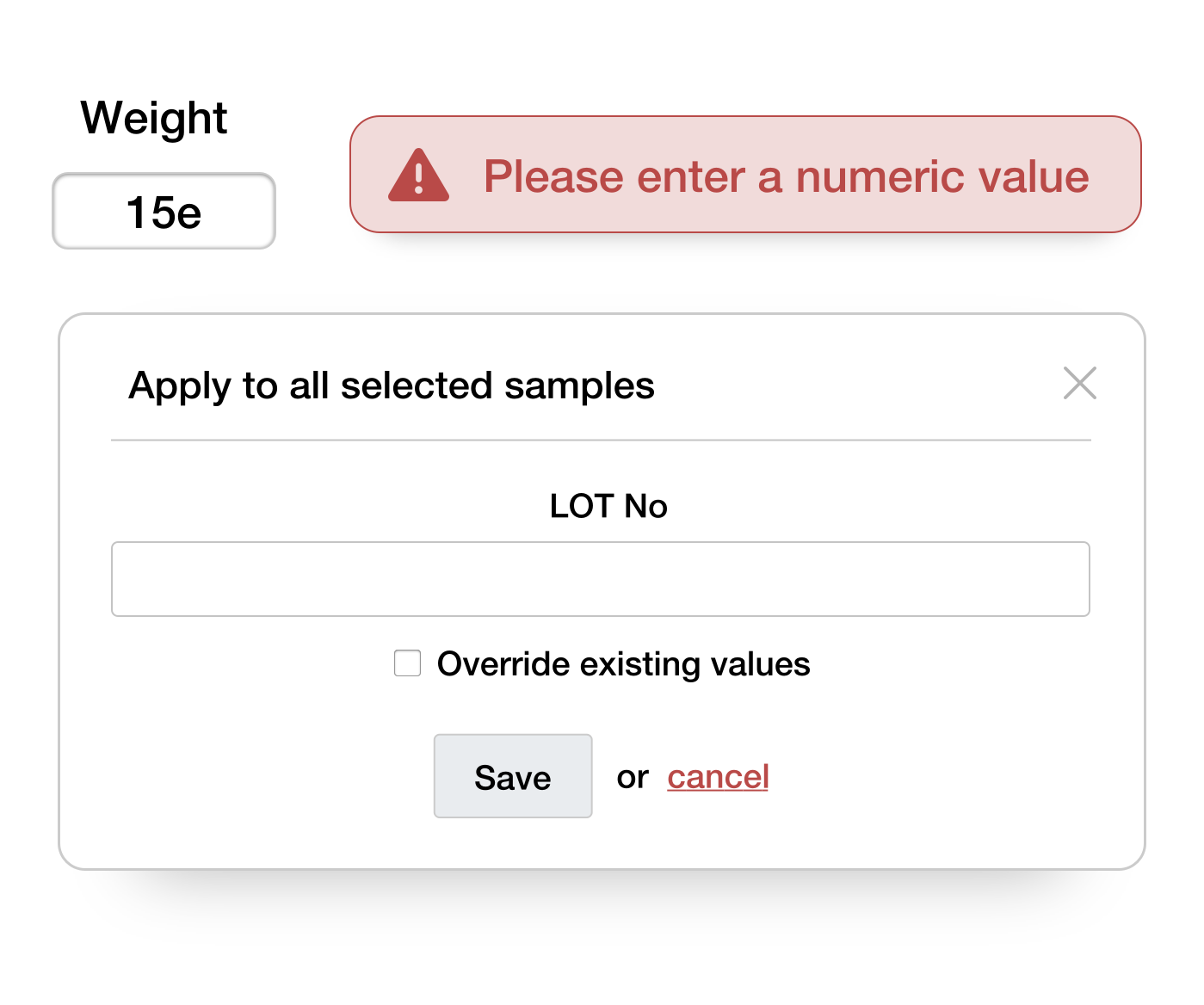
Prevents input errors
Data is validated during input and only needs to be entered once, drastically reducing input errors
-
Edit multiple samples at once
Enter redundant data like batch numbers for all relevant samples at once to save time
You want to learn more about data imports?
Book a free call with our experts:
Automate evaluation with threshholds, calculations and visualization
-
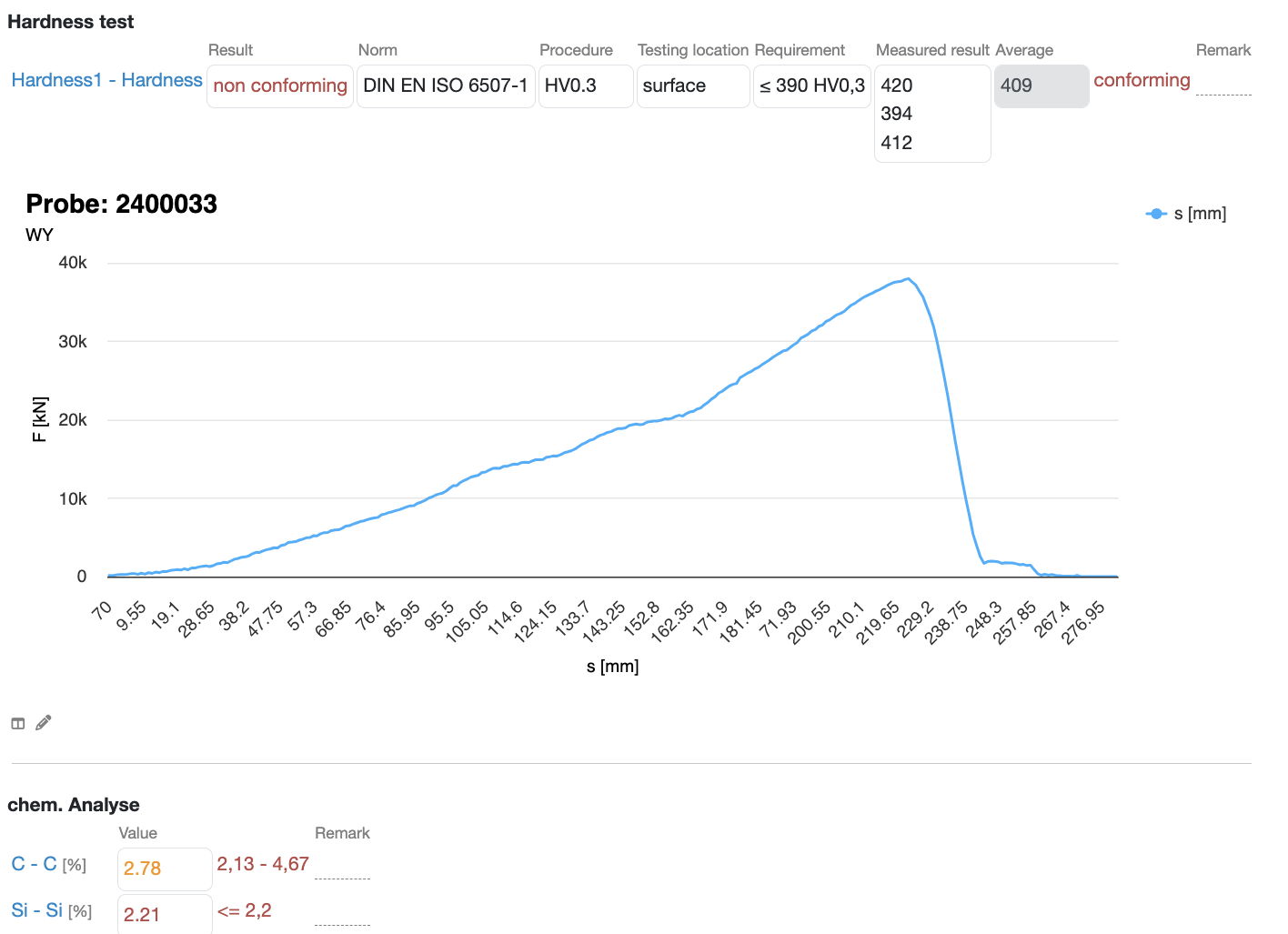
Multiple threshholds
Automatically check against threshholds for different interpretations and highlight nonconforming samples
-
Complex calculations
Build complex formulas using intuitive excel-like syntax to combine values from different parameters and transform them according to your needs
-
Detailed visualizations
Conveniently visualize your analysis data with flexible charts and diagramms
You want to learn more about sample evaluation?
Book a free call with our experts:
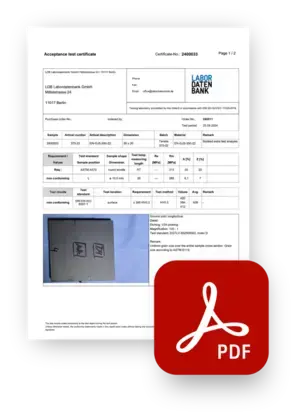
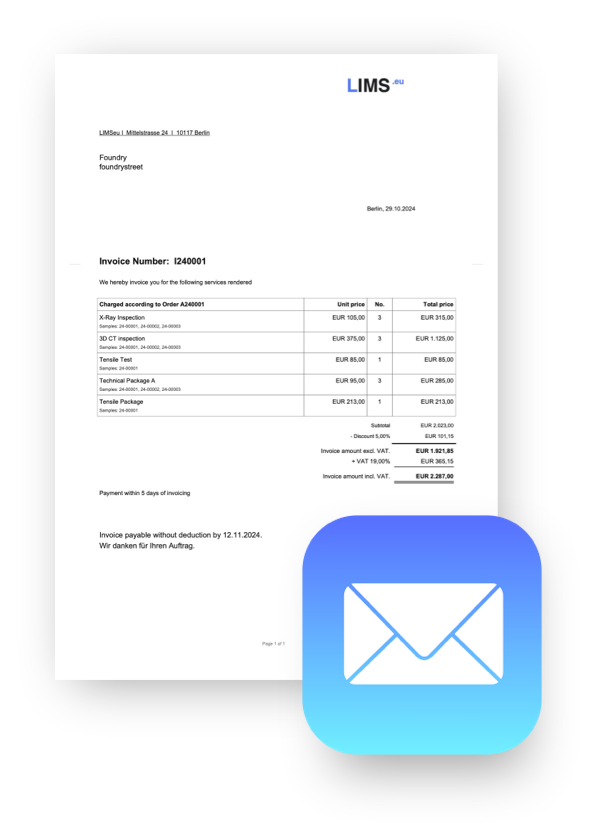
Create PDF reports with one click
-
Comprehensive reports - fully automated
Test results can be linked to coresponding text-modules, allowing you to automatically create comprehensive reports for every sample without having to write a single word.
-
Fully customizable, without coding
Create different report-templates containing all of your data, as well as images, charts and visualizations - all styled to your liking using our no-code editor
-
Easy documentation
All parties involved in the process can automatically be listed in the report
-
Tamper-proof & verifiable
Test reports are signed according to ETSI EN 319 142, can be validated online and are legally binding
You want to learn more about automated reports?
Book a free call with our experts:
Save time with integrated billing
Focus on your lab work and leave the paperwork to us
-
Automatically draw prices
based on the analyzed parameters of each sample
-
Customer specific pricing
can be configured for each parameter
-
Integrated invoicing
either fully automated or at the push of a button
-
XML Invoices (ZUGFeRD)
for seamless integration with accounting and ERP systems
You want to learn more about billing?
Book a free call with our experts:
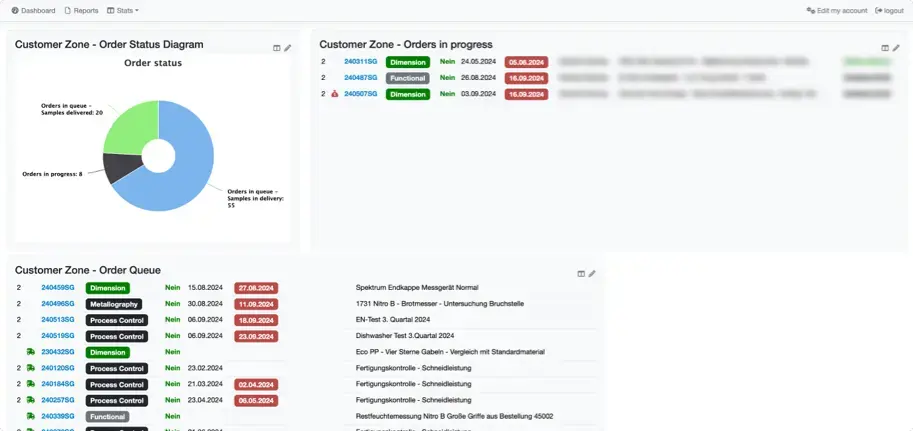
Secure client access to create orders and download PDFs
-
Secure web interface for each of your customers
Provide a private and secure webinterface to each of your customers, robustly secured via password and multi-factor authentication (MFA).
-
Convenient for you
Includes an order interface with customer-specific sample templates, completely removing the need for manual sample registration on your end.
-
Convenient for your customers
Customers can place new orders at the push of a button, track sample progress and securely access their results.
You want to learn more about the customer zone?
Book a free call with our experts:
Integrated ISO 17025 QM-Module
-
SOPs available right where you need them
in the currently released version - both during data collection and in the report
-
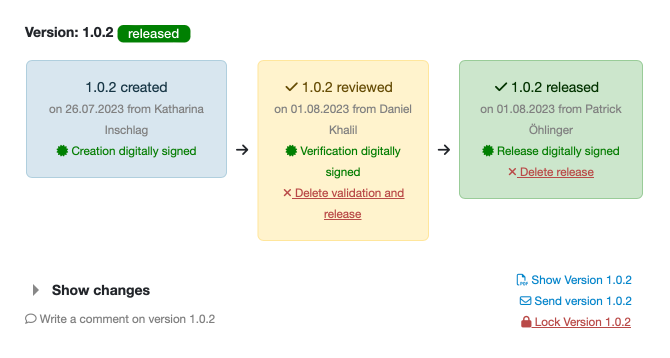
Version management
with creator, verifier and approver including digital signatures
-
Guided process
with automatic notification of the approver / inspector in the event of changes
-
Read confirmation
with automatic allocation of the qualification
You want to learn more about document control?
Book a free call with our experts:
-
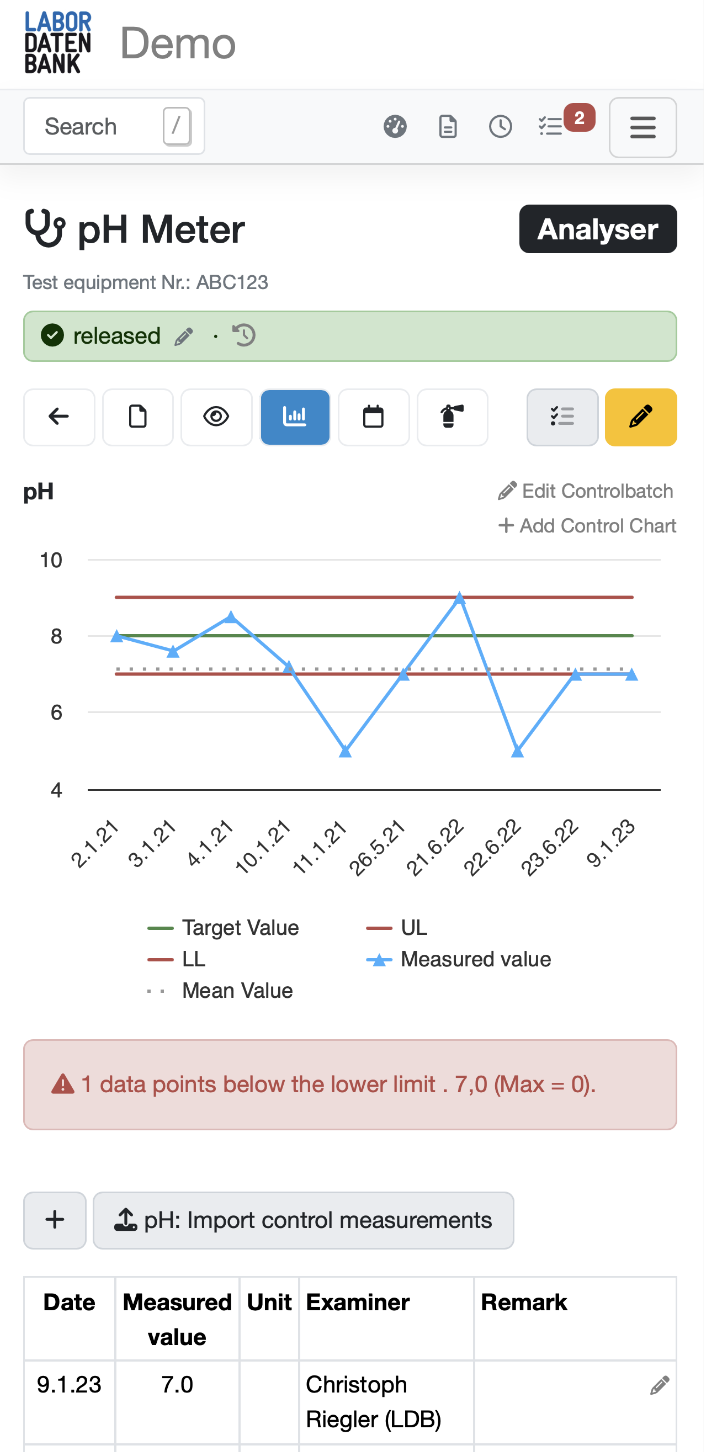
Test equipment data always available
Status of the test equipment available on the sample and in the report
-
Automatic reminders
for upcoming tasks such as calibration, verification, etc.
-
Relevant data for accreditation at a glance
certificates, maintenance data, etc
-
Control cards
Fixed or dynamic thresholds (Shewhart) with notifications in the event of deviation from the standard

You want to learn more about test equipment management?
Book a free call with our experts:
-
Batch tracing
Continuous tracking of which batch was used for which sample
-
Batch testing
Guided testing and approval process
-
Hazardous substance information
Comprehensive information on the materials used can be viewed at any time
-
Inventory management
Inventory data conveniently presented with automatic updates of stock on consumption
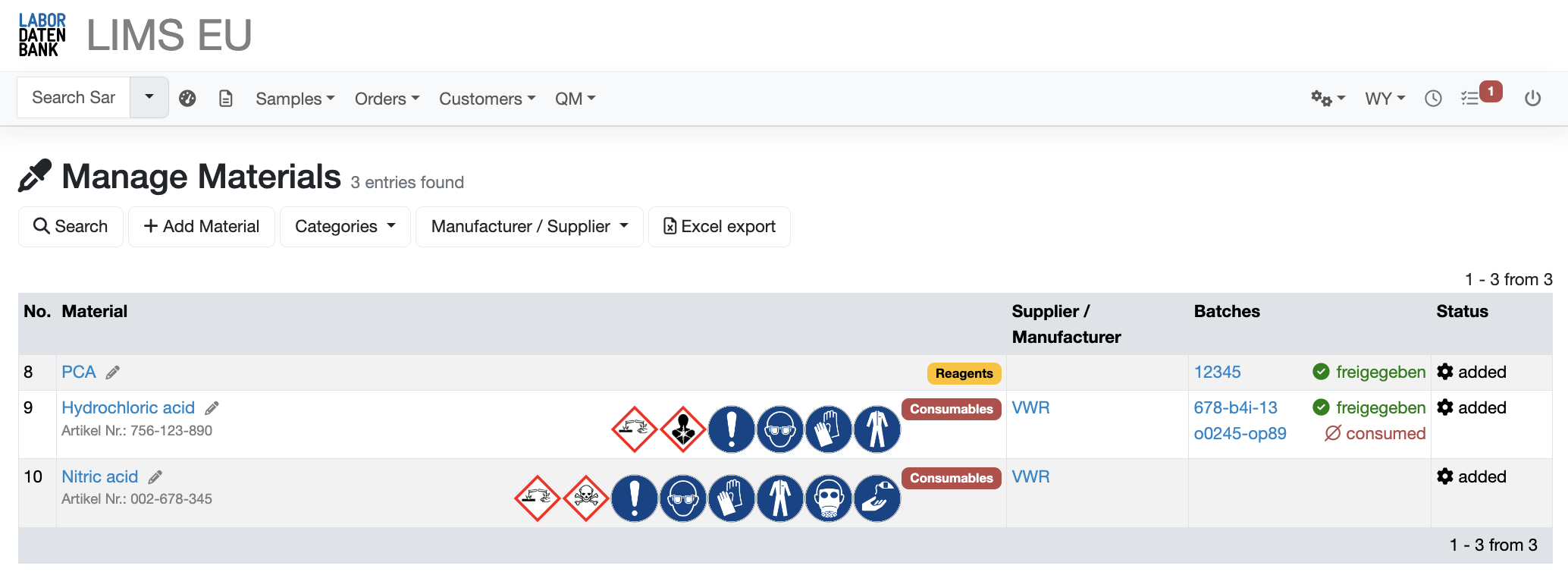
You want to learn more about material management?
Book a free call with our experts:
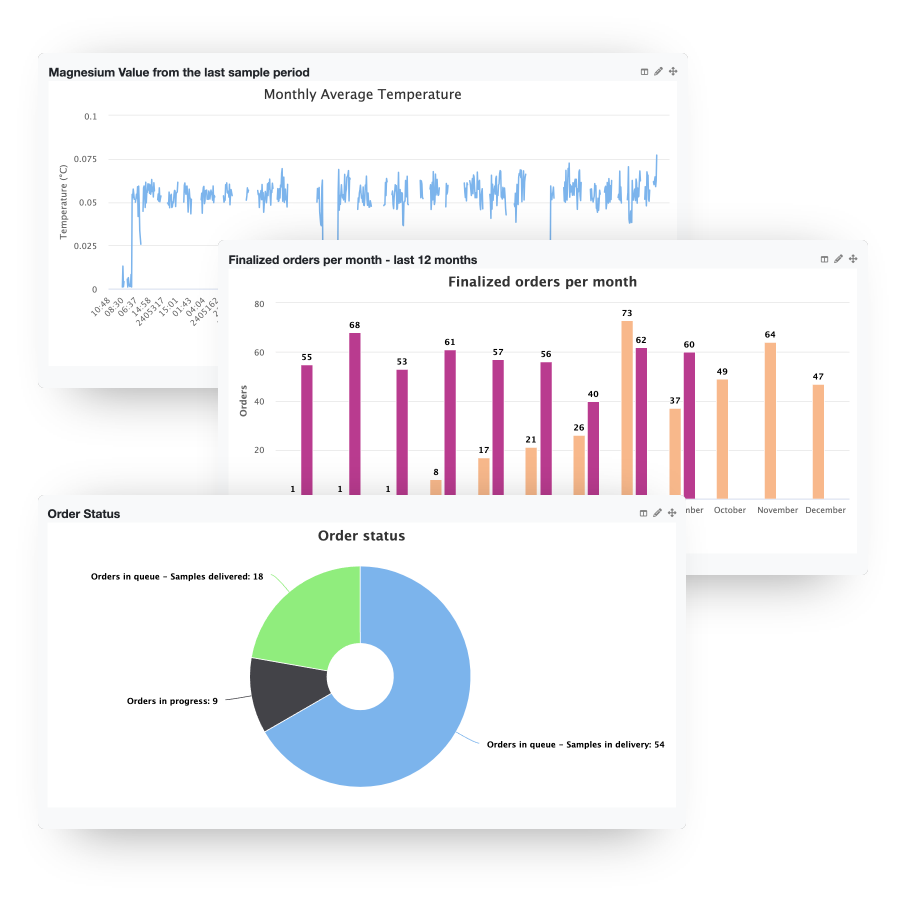
Visualize your data with graphs and dashboards
-
Comprehensive insights at a glance
Efficiently manage your lab using beautiful and comprehensive dashboards that allow you to gain insights into all relevant metrics for your lab at a glance
-
Intuitive data visualization
Analyze all the of the data your lab produces and make in useable and understandable using powerful charts and visualizations
-
User-specific dashboards
Create custom views and widgets tailored to specific users or user groups and restrict access using our comprehensive rights management